Not all breast cancers are created equal. Historically those patients with hormone-driven breast cancer had better outcomes compared to those with HER2+ breast cancer.
Human epidermal growth factor receptor 2 (HER2)/Neu is a kinase protein involved in normal cell growth. Sometimes there are errors in the genes that control the HER2 protein. This causes us to make too much of it helping breast cancer cells to grow quickly. This overexpression of HER2 occurs in 15-20% of breast cancers, sometimes in combination with hormone-positive disease, but sometimes independent of hormoneA chemical substance produced in the body that controls and regulates the activity of certain cells or organs. positivity. Expression of the protein on the cancer cell forms the basis of how we diagnose HER2+ breast cancer if the tumorA mass of cells that can be benign or malignant. strongly expresses the protein (3+) or moderately expresses (2 +) but shows signs that a cell is amplifying HER2 genes (FISH amplifiedFluorescence in situ hybridization (FISH) is a test that “maps” the genetic material in a person's cells. This test can be used to visualize specific genes or portions of genes. FISH testing is done on breast cancer tissue removed during biopsy to see if the cells have extra copies of the HER2 gene). There is growing interest in HER2 low tumors that express HER2 weakly (1+, 2+ but FISH negative).
When compared to breast cancers fueled solely by hormones such as estrogenA female sex hormone that is primarily produced by the ovaries. Its primary function is to regulate the menstrual cycle and assist in the production of secondary sex characteristics such as breasts. It may even play a role in the production of cancer cells in the breast tissue. and progesteroneA hormone that stimulates the uterus to prepare for pregnancy, produced mainly by the ovaries. Progesterone may play a role in certain breast cancers., HER2+ cancers will grow about 25% more aggressively, if left untreated. They grow faster, are more likely to metastasize and recur. Recurrences tend to happen earlier when compared to hormone-driven cancers, typically within 2-5 years. Brain metastases are more common, as well.
In the past, people with HER+ breast cancers had poor prognoses, but by targeting these protein receptors researchers have been able to develop treatments to equalize the gap.
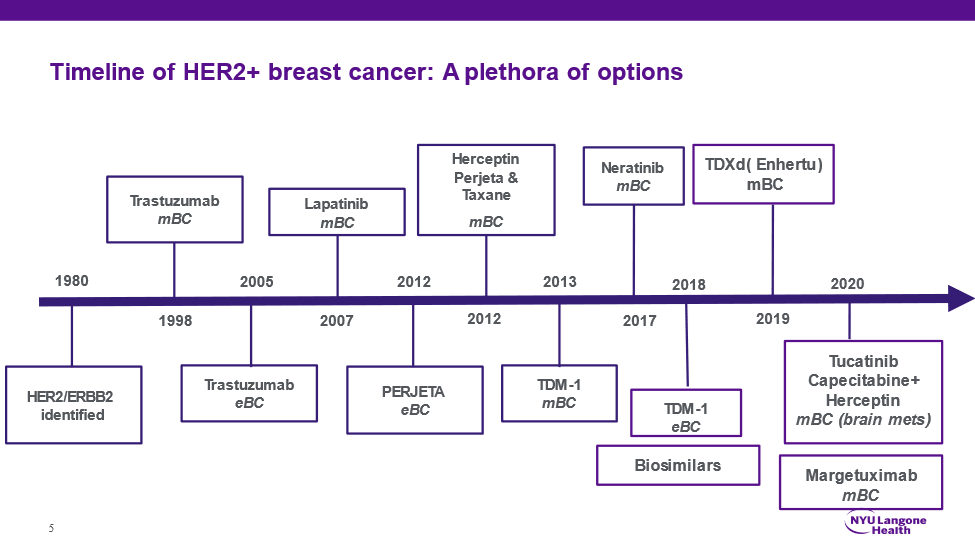
How have treatments evolved?
The evolution of HER2+ breast cancer treatments has focused on maximizing outcomes while minimizing toxicity. Although the HER2 geneA sequence in the DNA which can be passed down from parent to child. Genes helps determine physical and functional traits for the body. was identified in 1984, a targeted therapy to address the issue of protein overexpression was not available until the development of the monoclonal antibody treatment, Herceptin (Trastuzumab) in 1998. Prior to this drug, chemotherapyTreatment with drugs to destroy or slow down the growth of cancer cells. Often referred to as systematic treatment, because it acts throughout the body, as opposed to localized treatments, like surgery or radiation. was the standard of care for systemic treatmentA treatment like chemotherapy or hormonal therapy that affects the whole body or system, as opposed to localized treatment, such as surgery or radiation. of HER2+ breast cancer. When Herceptin was given alongside chemotherapy, not only was it well tolerated by patients, but recurrenceThe reappearance of the disease after it has been treated. In breast cancer, recurrence following primary breast cancer can be local (in the same place), regional (in surrounding tissue) or metastatic (in some other part of the body). rates decreased by 50% and mortality was cut by 30%. Outcomes improved further with the development of Pertuzumab, which works synergistically with chemotherapy and Herceptin.
Sometimes the receptor can be activated below the surface of the cancer cells, and the oral drugs known as tyrosine kinase inhibitors (TKI) work to block this activation downstream of receptor. Lapatinib, developed in 2007, was the first of its kind, with Neratinib and Tucatinib developed later. These drugs can penetrate and target brain metastases, which can be seen even in those patients with early disease, resulting in longer survival. The addition of this class of drugs to the standard regimen of chemotherapy and targeted therapy provides a double whammy to cancer growth.
Antibodies alone are only 20-25% effective as compared to the combination with chemotherapy, but researchers wanted to know how much chemo was really needed. The newest tools for blocking HER2 are antibody-drug conjugates (ADC), monoclonal antibodies combined with a low-dose chemotherapy drug. The antibody acts like a warhead honing in on the HER2 on cancer cells, delivering the chemo directly to them. Research reveals that with this approach patients are living longer with less side effects. TDM-1, ado-trastuzumab emtansine (Kadcyla), was the first ADC approved for HER2+ breast cancer . TDxD, trastuzumab deruxtecan (Enhertu), another more potent ADC has further moved the needle and improved outcomes for patients with metastatic HER2+. Most recently, HER2-low tumors with lower expression of the protein, have also been shown to respond to TDxD which has broadened use beyond just what we know as HER2+ cancer.
Where do we go from here?
The advancements that have been made in developing targeting therapies for HER2+ have helped to improve progression-free and overall survival for patients, with outcomes approaching levels seen in patients with hormone positive breast cancers. But, there is still work to be done. Currently, there are clinical trials studying if we can minimize or in some cases even avoid chemotherapy completely in women with early stage HER2+ breast cancer. This strategy of using response to these agents neoadjuvantlyUtilizing drugs, radiation therapy, or other means of supplemental treatment before cancer surgery or other primary cancer treatment. as a guide, minimizes toxicity without compromising outcomes . Brain metastases remain an area of unmet need as most traditional chemotherapies and even HER2 antibodies do not get into the brain. Newer oral agents and ADC’s have changed that as they show responses even in the brain. Studies are now looking at whether using these drugs early on can actually reduce the chance of developing brain metastases in the first place.
Participation in clinical trials is the only way we can continue to make advancements in breast cancer treatment. If you are interested in learning more about clinical trials that you may qualify for, you should speak with your doctor and visit https://clinicaltrials.gov/.
Sources
- Burstein HJ, et al. The Distinctive Nature of HER2-Positive Breast Cancers. N Engl J Med. 2005;353:1652-1654
- Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, Slamon DJ, Murphy M, Novotny WF, Burchmore M, Shak S, Stewart SJ, Press M. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002 Feb 1;20(3):719-26. doi: 10.1200/JCO.2002.20.3.719. PMID: 11821453
- Gonzalez-Angulo AM, Litton JK, Broglio KR, Meric-Bernstam F, Rakkhit R, Cardoso F, Peintinger F, Hanrahan EO, Sahin A, Guray M, Larsimont D, Feoli F, Stranzl H, Buchholz TA, Valero V, Theriault R, Piccart-Gebhart M, Ravdin PM, Berry DA, Hortobagyi GN. High risk of recurrence for patients with breast cancer who have human epidermal growth factor receptor 2-positive, node-negative tumors 1 cm or smaller. J Clin Oncol. 2009 Dec 1;27(34):5700-6. doi: 10.1200/JCO.2009.23.2025. Epub 2009 Nov 2. PMID: 19884543; PMCID: PMC2792998.
- Gianni L, Dafni U, Gelber RD, et al. for the Herceptin Adjuvant (HERA) Trial Study Team. Treatment with trastuzumab for 1 year after adjuvant chemotherapy in patients with HER2-positive early breast cancer: a 4-year follow-up of a randomised controlled trial. Lancet Oncol. 12(3):236-244, 2011.
- Slamon D, Eiermann W, Robert N, et al. for the Breast Cancer International Research Group. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 365(14):1273-83, 2011.
- Perez EA, Romond EH, Suman VJ, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. 32(33):3744-52, 2014.
- Annals of Oncology (2021) 32 (suppl_5): S1283-S1346. 10.1016/annonc/annonc741
- Masataka Sawaki, Tsuyoshi Saito, Shinichi Baba, Kokoro Kobayashi, et al. Evaluation of trastuzumab without chemotherapy as a postoperative adjuvant therapy in HER2-positive elderly breast cancer patients: Randomized controlled trial (RESPECT). Journal of Clinical Oncology 2018 36:15_suppl, 510-510