As a geneticInherited characteristics. counselor, I talk about geneticInherited characteristics. testing with individuals who have a personal or family history of cancer. We discuss the chance that their cancer or their relative’s cancer might be inherited, and review the option of testing to determine if they carry an inherited geneticInherited characteristics. mutation that may increase their risk of cancer. Occasionally, someone with a cancer diagnosis will say, “My doctor already did geneticInherited characteristics. testing”. In most cases, the tests already done were genomic, rather than geneticInherited characteristics. tests.
What does genetic testing look at?
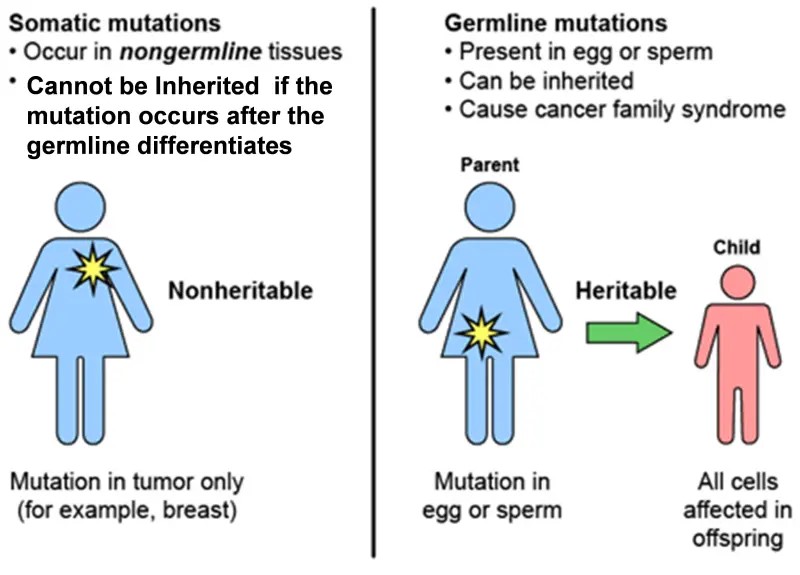
GeneticInherited characteristics. testing has traditionally referred to the analysis of genes that a person was born with. This is “germline” geneticInherited characteristics. testing, so called because what is being analyzed are genes as they were present in the germ cells (eggs and sperm) and therefore in the fertilized egg from which the embryo and fetus developed. A mutation present in the fertilized egg (a single cell) will be present in every cell that develops from that egg, and therefore, in (almost) every cell in a person’s body.
Hereditary cancer testing is geneticInherited characteristics. testing that is focused on finding an inherited mutation in a single geneA sequence in the DNA which can be passed down from parent to child. Genes helps determine physical and functional traits for the body., like BRCA1A gene which, when damaged or mutated, can put a person at a greater risk of developing breast and/or ovarian cancer. or BRCA2A gene which, when damaged or mutated, may put a woman at a greater risk of developing breast and/or ovarian cancer. This gene is also thought to raise the risk for breast cancer in men.. GeneticInherited characteristics. testing is available to individuals with or without cancer, and can be performed on multiple different tissues from the body, blood or saliva being the most common. Testing for a geneA sequence in the DNA which can be passed down from parent to child. Genes helps determine physical and functional traits for the body. mutation associated with hereditary breast cancer is typically offered with a cancer diagnosis at age 50 or younger, triple negative cancer, personal history or close relative with ovarian, pancreatic or metastatic prostate cancer, male breast cancer, Ashkenazi Jewish ancestry, or known mutation in the family. The number of genes analyzed varies, but may be anywhere from 1 to around 80 or more, depending on what is known about personal and family history, and how much information is desired.
How genetic testing helps with breast health planning
If geneticInherited characteristics. testing identifies a germline mutation, this provides the individual and their healthcare team with information about the risk to have cancer in the future, type of cancer, and the chance for close relatives to have the same mutation. People who discover they are carriers of harmful geneticInherited characteristics. mutations prior to a cancer diagnosis can work with a medical team to create a risk reduction plan. If cancer develops, knowing there is a germline mutation may help oncologists determine the best course of treatment. For example, an individual with a germline BRCA1A gene which, when damaged or mutated, can put a person at a greater risk of developing breast and/or ovarian cancer. or BRCA2A gene which, when damaged or mutated, may put a woman at a greater risk of developing breast and/or ovarian cancer. This gene is also thought to raise the risk for breast cancer in men. mutation may be eligible for treatment with a PARP inhibitor after other cancer treatments are completed.
Germline vs Somatic (Sporadic) Mutation
What does genomic testing focus on?
Genomic testing is focused on finding acquired mutations in multiple genes in cancer tissue. (Acquired mutations are also referred to as somatic or sporadic mutations. Somatic mutations cannot be passed on to children.) In most of the cells in a body, genes are unchanged from birth. However, geneticInherited characteristics. changes, or damage to DNAThe part of every cell that carries out genetic information on cell growth, division, and function., can happen after birth, due to aging and environmental exposures. Common exposures that cause geneticInherited characteristics. changes, or somatic mutations, are sunlight, use of tobacco, use of alcohol, occupational exposures (such as asbestos or benzene) and viruses (such as HPV or HIV). Somatic changes do not always lead to cancer. Cells with DNAThe part of every cell that carries out genetic information on cell growth, division, and function. damage often die naturally. But a cell with DNAThe part of every cell that carries out genetic information on cell growth, division, and function. damage that acquires more damage in multiple genes, and creates more cells with the same abnormal DNAThe part of every cell that carries out genetic information on cell growth, division, and function., may develop into a tumorA mass of cells that can be benign or malignant..
What does genomic testing have to do with breast cancer?
Each tumorA mass of cells that can be benign or malignant. has a unique pattern of DNAThe part of every cell that carries out genetic information on cell growth, division, and function.. Analysis of this pattern creates a genomic tumorA mass of cells that can be benign or malignant. profile, with information that enables oncologists to determine a course of treatment that will target the features of the cancer cells that are allowing them to multiply. Oncotype and Mammaprint are two genomic tumorA mass of cells that can be benign or malignant. profiling tests that may be ordered as part of analysis of breast tumors. These tests can help predict whether chemotherapyTreatment with drugs to destroy or slow down the growth of cancer cells. Often referred to as systematic treatment, because it acts throughout the body, as opposed to localized treatments, like surgery or radiation. will be beneficial.
In addition to detecting somatic mutations, genomic testing may also analyze tumorA mass of cells that can be benign or malignant. tissue to determine the activity or expression of certain genes, or biomarkersA distinct biochemical, genetic, or molecular characteristic or substance that is an indicator of a particular biological condition or process., that can be targeted with a specific drug or drugs. The identification of and attention to specific DNAThe part of every cell that carries out genetic information on cell growth, division, and function. patterns within a tumorA mass of cells that can be benign or malignant., and creation of targeted therapies, is referred to as personalized medicine, or precision medicine. With the development of personalized and targeted treatment, cancer treatment is no longer “one size fits all.” Fewer patients need chemotherapyTreatment with drugs to destroy or slow down the growth of cancer cells. Often referred to as systematic treatment, because it acts throughout the body, as opposed to localized treatments, like surgery or radiation., or may be able to receive a type of chemotherapyTreatment with drugs to destroy or slow down the growth of cancer cells. Often referred to as systematic treatment, because it acts throughout the body, as opposed to localized treatments, like surgery or radiation. that is less toxic.
While genomic tumorA mass of cells that can be benign or malignant. testing is focused on identification of somatic mutations, it occasionally identifies a mutation that is possibly germline. Individuals who have a possible germline mutation on tumorA mass of cells that can be benign or malignant. testing are referred for geneticInherited characteristics. testing to rule out or confirm that it is germline. Using a combination of geneticInherited characteristics. and genomic information, oncologists are able to better predict which treatments will be effective and well tolerated.